Thyroid nodules are very common. Approximately 50% of the population have thyroid nodules on ultrasound examination. Most thyroid nodules are benign while some are malignant. The incidence of thyroid cancer is 13 per 100,000 per year. About 1 in 100 people develop thyroid cancer over the course of their lifetime. It is three times more common in women.
To determine which thyroid nodules are cancerous, ultrasound and FNA cytology of suspicious thyroid nodules are the mainstay of investigation. Please see the article entitled “How to manage a patient with a thyroid nodule” in the June edition of GP Voice. About 10% of thyroid FNAs are reported as Bethesda 3 (atypia of undetermined significance) and a further 10% are reported as Bethesda 4 (follicular neoplasm), but only about 16–23% of these patients actually have thyroid cancer.3 It is common practice, however, to offer patients with Bethesda 3 and 4 cytology surgery in the form of a diagnostic thyroid lobectomy. Although this approach detects and treats thyroid cancer in 16–23% of people in this category, 77–84% of people have an operation with little or no therapeutic benefit.
To help further determine which patients are more likely to have cancer, molecular analysis of FNA specimens can be performed. This involves testing the DNA and RNA of the cells obtained in the FNA specimens. These tests look specifically for mutations in genes associated with thyroid cancer. The prevalence of some of the common genetic mutations are listed below.
Papillary thyroid carcinoma: prevalence of genetic mutations

Follicular carcinoma: prevalence of genetic mutations

In America, Thyroseq® and Affirma® are two of the common molecular tests looking at genetic mutations seen in thyroid cancers. Affirma® looks at 593 genes associated with thyroid cancer. Using Affirma® 2/3 of patients with Bethesda 3 and 4 nodules are reclassified as benign.
Thyroseq® looks at 112 genes associated with thyroid cancer. Similarly, Thyroseq® is able to stratify patients with Bethesda 3 and 4 nodules into low, intermediate and high risk of having thyroid cancer. Low risk patients can be observed. Thyroid lobectomy is recommended for intermediate risk patients and total thyroidectomy is recommended for high-risk patients with aggressive types of thyroid cancer.
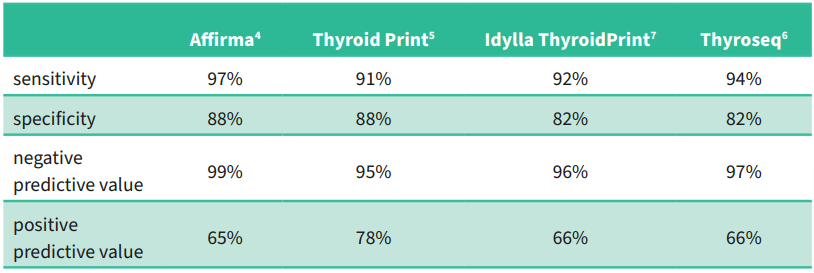
It is important for the reader to realise that as the prevalence of a disease increases in a population, the positive predictive value increases and the negative predictive value decreases.
In New Zealand, Thyroseq® molecular analysis of FNA specimens of thyroid nodules is available, but only if the FNA specimens are sent to Sullivan Nicolaides Pathology laboratory in Brisbane who in turn send it to the Sonic laboratory in New York, USA. The turnaround time is about four weeks. The cost to the patient is AU$2,100 (~NZ$2,300) and this is not covered by the patient’s health insurance.
In the near future, Idylla ThyroidPrint® molecular analysis of FNA specimens of thyroid nodules will be available in New Zealand with results available in 24 hours. Idylla ThyroidPrint® looks at 10 epithelial and stromal cell target genes associated with thyroid cancer. It stratifies risk into low (observe) and high (operate).
The American Thyroid Association (ATA) guidelines recommend three options for the management of patients with Bethesda 3 thyroid nodules:
- Diagnostic thyroid lobectomy
- Close observation
- Molecular analysis of Bethesda 3 nodules.
Molecular analysis of FNA specimen needs to be considered alongside the history, examination findings, thyroid function tests, ultrasound findings and FNA cytology. Molecular analysis of FNA specimen gives valuable additional information to help decide which of the above three options is most appropriate.
In summary, molecular analysis of thyroid nodules is a useful adjunct to ultrasound and cytology in the evaluation of thyroid nodules for malignancy. It is particularly useful in patients with indeterminate cytology (Bethesda 3 and 4) and may reduce the number of diagnostic thyroid lobectomies. For further information on molecular testing of your patient with a thyroid nodule, contact Dr Francis Hall on francis@drfrancishall.co.nz
References:
1. Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. (2017) Journal of the American College of Radiology : JACR. 14 (5): 587–595. doi:10.1016/j.jacr.2017.01.046
2. Middleton WD, Teefey SA, Reading CC, et al. Multi institutional Analysis of Thyroid Nodule Risk Stratification Using the American College of Radiology Thyroid Imaging Reporting and Data System. (2017) American Journal of Roentgenology. 208 (6): 1331–1341. doi:10.2214/AJR.16.17613
3. Ali SZ, et al. The 2023 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2023; 33: 1039–1044.
4. Nasr C, et al. Real-world performance of the Affirma genomic sequencing classifier (GSC)-a meta analysis. JCEM 2022; 108: 1526–1532.
5. Steward DL, et al. Performance of a multigene genomic classifier in thyroid nodules with indeterminate cytology. A prospective blinded multicenter study. JAMA Oncol. 2019; 5: 204–2012. doi:10.1001/jamaoncol.2018.4616.
6. Zafero M, et al. A thyroid genetic classifier correctly predicts benign nodules with indeterminate cytology: two independent, multicenter, prospective validation trials. Thyroid 2020; 30: 704–712.
7. Unpublished data (internal analysis based on 132 indeterminate samples compared to the surgical gold standard).
8. Haugen BR, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2016; 26: 1–133.

